Breakthrough Study: Emeramide Rapidly Reduces Mercury in Gold Miners, Offering a Safe, Effective Detox Treatment.
The Preventive Dental Health Association (PDHA) is raising awareness of a study recently published in the journal Biometals, which unveiled a significant advancement in the treatment of mercury intoxication. This upcoming therapy offers a potential lifeline to dental personnel working with mercury and to people with mercury amalgam dental fillings.
The human clinical trial, led by a team of distinguished researchers, focused on the effectiveness of Emeramide, [NN′bis-(2-mercaptoethyl) isophthalamide (NBMI)], a new lipophilic chelating agent with strong antioxidant properties. Emeramide was found to rapidly, safely, and effectively reduce mercury levels in the human body, which is a breakthrough given the absence of effective treatments for mercury poisoning.
The study involved 36 Ecuadorian gold miners with elevated urinary mercury concentrations. Participants were assigned to three groups to receive either 100 mg or 300 mg of Emeramide per day or a placebo for a 14-day period. The study meticulously monitored and analyzed changes in mercury concentrations in both plasma and urine based on the amount of Emeramide each participant received.
The results were striking. The gold miners had a significant reduction in the many symptoms from mercury intoxication. The more Emeramide received, the greater reduction in mercury levels was observed in both urine and plasma of participants receiving Emeramide treatment, compared to those on placebo. Notably, these reductions remained significant 30 days post-treatment, indicating that Emeramide entered the cell’s cytoplasm and bound intracellular mercury, which represents the bulk of body mercury. Specifically, there was a 30% reduction in urinary mercury concentrations per mg of Emeramide per kg of body weight per day.
The study hypothesizes that Emeramide’s effectiveness is attributed to its ability to cross cellular bio-membranes and form stable, non-toxic complexes with mercury, thereby facilitating its removal from the body.

These findings indicate that Emeramide therapy effectively and rapidly reduces mercury levels while maintaining this reduction over time by irreversibly binding to the bulk of mercury body burden, which is intracellular and not available to current chelation methods. This is significant because many existing chelation therapies use charged chelators like DMSA or DMPS, which often result in redistribution and rebound increases in heavy metal concentrations in the body.
The safety of Emeramide was also a focal point of the study. Previous animal model studies and the human clinical trial both indicated a strong safety profile for Emeramide, with no serious drug-related adverse effects observed. This positions Emeramide as a viable and safe option for mercury detoxification in humans.
The implications of this research are profound. Mercury exposure is a pressing global health concern, with numerous populations worldwide with mercury intoxication due to environmental and occupational exposure. Current treatment options are limited, and the discovery of Emeramide’s efficacy in reducing mercury levels presents a potential game-changer in the field of toxicology and public health. Additionally, it offers new hope for affected populations, such as those with mercury amalgam dental fillings. Notably, this group represents over 120 million Americans.
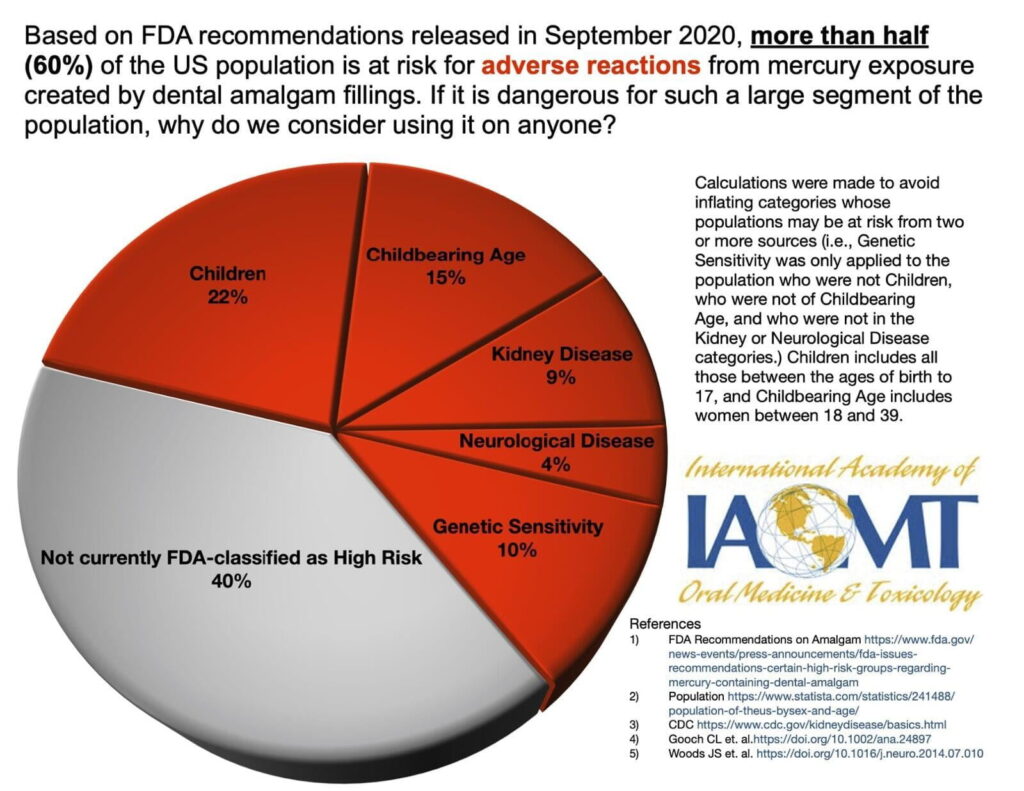
In 2020 the FDA found that certain groups may be at greater risk for potential harmful health effects of mercury vapor released from amalgam fillings. Those likely affected by mercury from fillings are women of childbearing age, nursing mothers, newborns, infants, children under 6 years of age, people with neurological diseases, impaired kidney function, and those with a heightened sensitivity (allergy) to mercury. These susceptible populations make up roughly 60% of the U.S. population.

When PDHA inquired about the remaining steps to bring Emeramide to the market, Emeramed chief scientist Boyd Haley, PhD, explained, “All previous studies on NBMI have consistently shown the product’s safety and effectiveness for multiple metals. We are in the process of securing funding to conduct a phase 3 clinical trial for mercury in Colombia. Once the trial is finished, we will submit the results to the FDA to seek marketing authority.”
In conclusion, the study presents Emeramide as a highly effective and safe treatment for reducing mercury levels in the human body. Its potential impact on global health, especially in mercury-exposed populations, is immense. As the study authors advocate, further research and clinical trials are warranted to fully explore and harness the therapeutic powers of Emeramide in combating mercury intoxication.

